Milestone 1
Structural basis of the precursor-protein surface interactions
In this milestone, we aim at understanding why a polycationic polypeptide or protein can promote the formation of condensed inorganic networks from soluble precursors.
Do charged residues exert a catalytic action? Or is their role only linked to the initial interaction with the precursor?
Paper #1:
We performed non-reactive MD simulations of lysozyme in the presence of silicic acid, from the concentration that is used in the polycondensation reaction up to 100 times more concentrated, a condition that is not attainable experimentally. These simulations confirm the model of the electrostatic pooling in the polycondensation of silica in the presence of lysozyme.
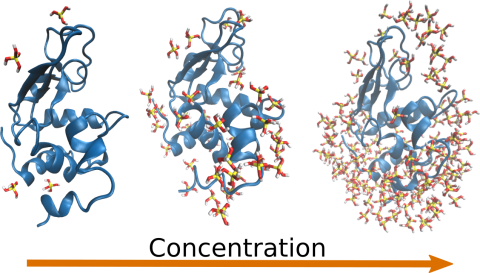
On these grounds, we can postulate that the protein scaffold that harbors several charged sidechains in an orderly fashion exerts the same effect as a LLPS coacervate: in the protein, the charge is strongly concentrated and localized, as is in the coacervates of polycationic molecules, and thus contribute to concentrate the precursor molecules, in turn increasing the reaction rate. This picture thus reconciles nicely the experimental observations that LLPS is required for silicification peptides and not for polycationic proteins. As a last remark, this conclusion is reminiscent of the view of proteins as colloids. The role of specific sidechains appears blurred in the present example.
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.202401249